Laboratory for Innate Immune Systems
Current research
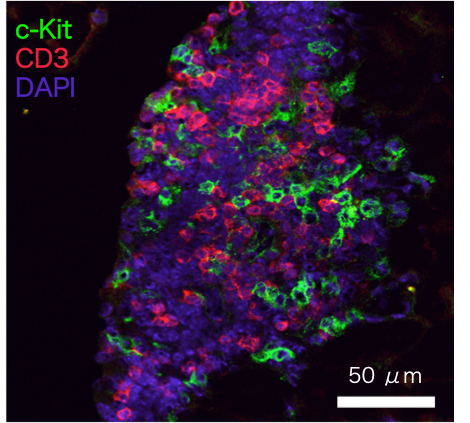
Origin of FALC
FALC is a unique lymphoid cluster that we discovered in adipose tissue, which contains a number of immune cells including ILC2. Blood vessels and lymphatic vessels are found in FALC, suggesting that cells enter and exit the tissue. FALC exists in aly/aly or RORγt deficient mice, which are known to lack all lymph nodes, implying that this lymphoid tissue is distinct from typical lymph nodes. Furthermore, mice deficient in gc, a common subunit of receptors for IL-2, -4, -7, -9, -15 and 21, lack FALC, suggesting that gc plays a critical role in FALC development. We study the mechanisms that mediate the formation of FALC in adipose tissues as well as mechanisms that control migration of immune cells into FALC.
Role of ILC2 in allergic inflammation
We have shown that ILC2 play an important role in allergic inflammation in the lung. We have also shown that ILC2 and other innate immune cells such as basophils and eosinophils interact with each other to induce allergic inflammatory responses. Furthermore, our studies demonstrated that ILC2 play a pivotal role in steroid resistance in airway inflammation, through the action of IL-2, IL-7 and TSLP, all of which are Stat5 activators. We have recently been focused on the regulation mechanisms of cytokine production from ILC2 during allergic inflammation. In addition, we want to further clarify the functional interaction of ILC2 with other immune cells.
Role of ILC2 in the homeostasis of adipose tissues
ILC2 were originally found in the FALC structure in adipose tissues. It has been shown that numerous immune cells, including macrophages, play important roles in the homeostasis of adipose tissues and that failure of homeostatic regulation in adipose tissues leads to various diseases including insulin resistance and type 2 diabetes. ILC2 constitutively produce the type 2 cytokines IL-5 and IL-13 that are known to act on various immune cells such as macrophages and eosinophils. We study the function of ILC2 in obesity-induced inflammatory responses in adipose tissues.
Physiological roles of ILC2 in various peripheral tissues
ILC2 are located in various peripheral tissues. They are known to be tissue-resident cells that do not circulate in the blood stream, at least in the steady state. Therefore, we are investigating the specific roles of ILC2 in various tissues other than adipose, such as lung, skin, intestine, pancreas and liver. We utilize tissue-specific disease models to study the function of ILC2 during inflammation.
Cell fate decision of ILC2
Similar to other lymphocytes such as T cells, B cells and NK cells, ILC2 are derived from common lymphoid progenitors (CLP) via several specific progenitors including EILP, CHILP and PLZF+ ILCP. However, the environmental factors that regulate the commitment from common progenitors toward ILC lineage and the site and stromal cells that support ILC2-specific differentiation are not fully understood. We established an in vitro ILC2 differentiation system and revealed that the quantity of IL-7 and Notch signaling differentially determine the fate of CLP to ILC lineage. In addition, we identified ILC progenitors and immature ILC2 in the fetal peripheral tissues. Furthermore, we found that PDGFRα+gp38+ mesenchymal cells in the adipose tissue support ILC2 differentiation and maturation form ILC progenitors, suggesting that terminal differentiation of tissue-resident ILC2 occurs in the periphery with the aid of PDGFRα+gp38+ mesenchymal cells. We are currently investigating the contribution of PDGFRα+gp38+ cells to ILC2-specific development to understand how and when these mesenchymal cells regulate ILC2 differentiation, maturation and maintenance in the peripheral tissues.
