Collaborative research by RCAI teams led by Sidonia Fagarasan and Shohei Hori resulted in a surprising discovery. Foxp3+ T cells, called Tregs because of their ability to suppress immune responses, can differentiate into follicular B helper T cells in the gut.
The transcription factor Foxp3 is a marker for Tregs, which play a central role in preventing pathological immune responses and ensuring tolerance to self- and innocuous environmental antigens. However, Hori recently reported unstable expression of Foxp3 and developmental plasticity of Foxp3+ T cells.
IgA in the gut is critical for maintaining the immune system homeostasis, by controlling the vast community of intestinal bacteria. IgA is mostly produced by B cells in gut Peyer's patches (PP), and requires the presence of follicular B helper T cells (TFH). These B cells and TFH co-localize in a specialized structure, the germinal center (GC), where B cells undergo isotype class switching and somatic hypermutation, upon induction of activation-induced cytidine-deaminase (AID). However, the origin of TFH cells in PP was unknown.
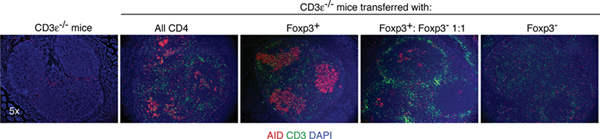
To investigate the origins of TFH in PPs, the teams transferred Foxp3+ and/or Foxp3- T cells into T cell deficient mice. To their surprise, typical GCs were formed moslty when Foxp3+ T cells were transferred. Furthermore, the induction of GCs (figure) and generation of IgA-producing cells in PPs were more effective than that induced by unfractionated T cells or a 1:1 mixture of Foxp3+ and Foxp3- T cells.
A large fraction of the transferred Foxp3+ T cells lost Foxp3 expression and preferentially localized to the B cell follicles. These cells expressed chemokine receptors, transcription factors, and cytokines critical for migration, generation and function of TFH. Thus, Foxp3 expression is plastic and cells that previously expressed Foxp3 are effective precursors for TFH cells.
To determine whether B cells are required for the differentiation to TFH cells, they transferred Foxp3+ T cells to mice deficient in both T and B cells. In the absence of B cells, Foxp3+ T cells failed to differentiate into TFH cells, although Foxp3 expression was down regulated. Thus, differentiation of Foxp3+ T cells into TFH cells requires interaction with B cells while down-regulation of Foxp3 expression does not.
Foxp3+ T cells were converted into TFH cells only in PPs. When they transferred Foxp3+ T cells into T cell deficient mice, neither TFH cells nor GCs could be detected in spleen or lymph nodes after immunization. In contrast, Foxp3- T cells generated GCs in the spleen.
The study suggests that, depending on the environment, TCR stimulation induces either "suppressor" or "helper" T cells. The studies have implications for understanding how suppression of inflammatory reactions and induction of IgA synthesis occur in the gut.
Figure: T cell deficient mice were untreated or transplanted with the indicated cell types. Four weeks later, sections of Peyer's patches were examined by immunofluorescence microscopy. AID, activation induced cytidine deaminase, a marker for GC B cells undergoing isotype switching/somatic hypermutation (red); CD3, a T cell marker (green); DAPI, a DNA stain to reveal cell nuclei (blue).
ORIGINAL RESEARCH PAPER
Tsuji, M., * Komatsu, N.,* Kawamoto, S.,* Suzuki K., Kanagawa, O., Honjo, T., Hori, S.,† Fagarasan, S.† Preferential Generation of Follicular B Helper T (TFH) Cells from Foxp3+ T Cells in Gut Peyer's Patches. Science 323, 1488-1492 (2009)