Projects
SEAPharm for establishment of stratified medicine in Asia
In 2012, RIKEN established the South East Asian Pharma-cogenomics Research Network (SEAPharm) together with five other Asian countries (Korea, Indonesia, Malaysia, Taiwan, and Thailand). Membership has been steadily increasing, with Singapore joining the team in 2014, Vietnam in 2016, Nepal, Laos and the Philippines in 2017, and Brunei and Myanmar in 2018. The aims of the collaboration are to identify genomic biomarkers associated with adverse drug reactions, such as severe cutaneous adverse drug reactions (ADRs), including Stevens-Johnson syn-drome (SJS), toxic epidermal necrolysis (TEN) and hepatic injury, to provide technical assistance and training of young researchers from the SEAPharm member countries, and to hold international seminars and workshops.Recently, SEAPharm has started a new project involving next-generation sequencing (NGS) of about 2,000 genomic DNA samples from 12 countries to clarify the genetic diversity of 100 pharmacokinetics-related genes in individuals from Southeast Asia, Sothern Asia, Middle East and Southern Europe. RIKEN IMS is responsible for the targeted sequencing using a PKSeq panel developed by RIKEN and reported substantial genetic vari-ations in drug-metabolizing enzyme and drug transporter genes among Asian populations. These findings can account for inter-ethnic variabilities in drug response phenotypes, and are leading to acceleration of further pharmacogenomic investigations and genotype-guided drug therapies in clinical practice.
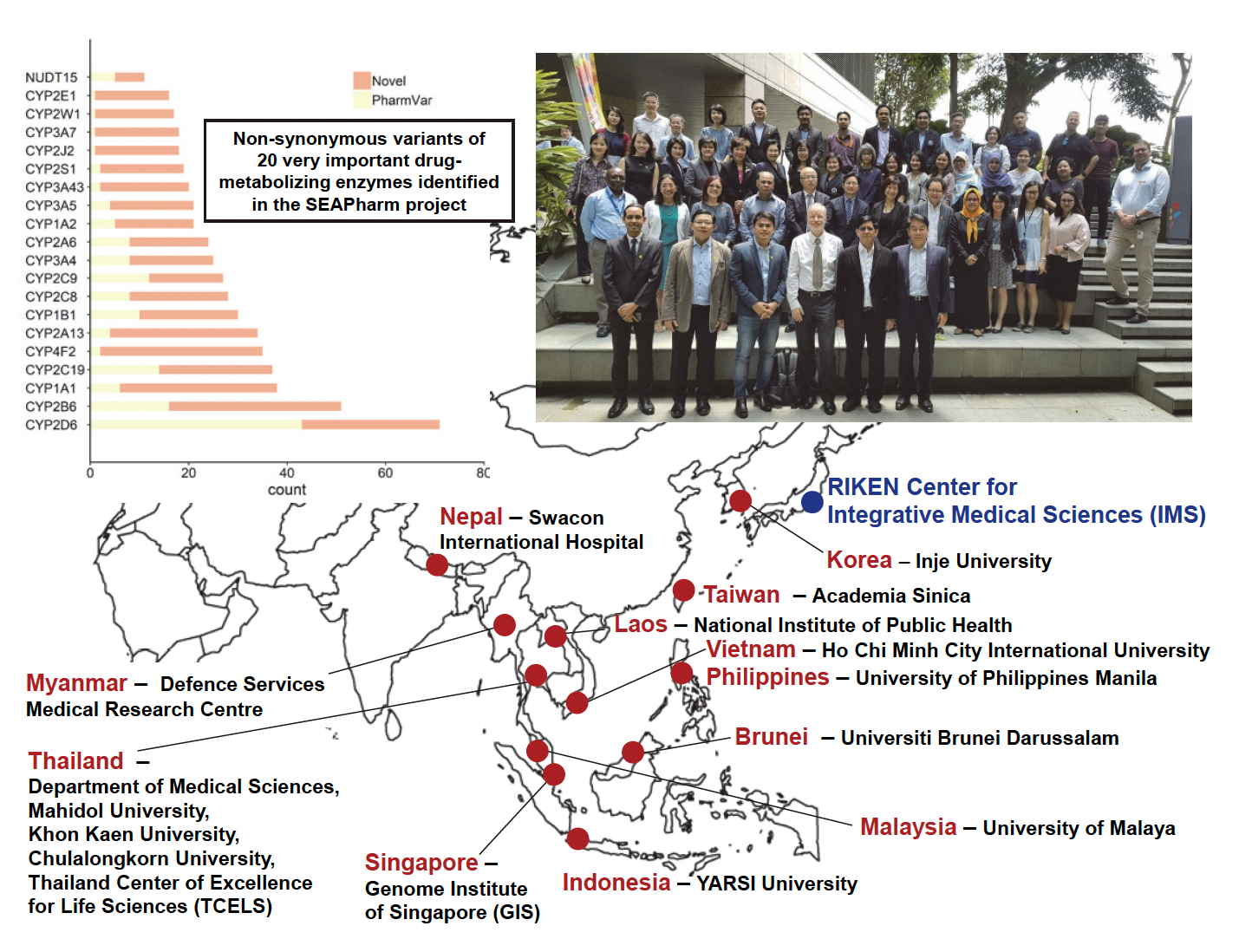
Figure: Members of the South East Asian Pharmacogenomics Research Network
(SEAPharm)Please visit https://www.facebook.com/SEAPHARM/