Projects
iPS Project
Induced pluripotent stem (iPS) cells possess tremendous therapeutic potential in many areas, including regenerative medicine and immune therapy. We have begun an activity to apply iPS technology to both mouse and human immunology research and to develop therapeutics. On a collaborative basis with individual RCAI-IMS research laboratories, the core facility for iPS research is engaged in developing efficient protocols to reprogram various types of lymphocytes into iPS cells, as well as to induce differentiation of iPS cells into a variety of lymphoid lineage cells. This activity is partly supported by the Research Center Network for Realization of Regenerative Medicine from the Japan Agency for Medical Research and Development (AMED) and CREST, Japan Science and Technology Agency.
The facility has operated an IMS Cell Manufacturing Unit (CMU) to produce iPS-Vα24+iNKT cells under GMP (Good Manufacturing Practice)/GCTP (Good Gene, Cellular, and Tissue-based Products Manufacturing Practice) guidelines. The safety of these iPS-Vα24+iNKT cells was confirmed by preclinical studies. The facility has been finishing PMDA (Pharmaceuticals and Medical Devices Agency) consultation for eventual clinical trials of iPS-Vα24+iNKT cell-mediated head and neck cancer immunotherapy.
Differences in human leukocyte antigen class I (HLA-I) genes can cause rejection in an allogeneic transplantation situation. To address this concern, this year, the facility aimed to prepare Beta-2-Microgloblin (B2M) gene-disrupted human iPS-Vα24+iNKT cells using CRISPR/Cas9 and then they observed a distinct reduction in alloreactive CD8+ T cell-mediated killing (Figure 1). These results suggest that B2M-disrupted iPS-Vα24+iNKT cells will be more useful in allogeneic transplantation due to their longer half-life.
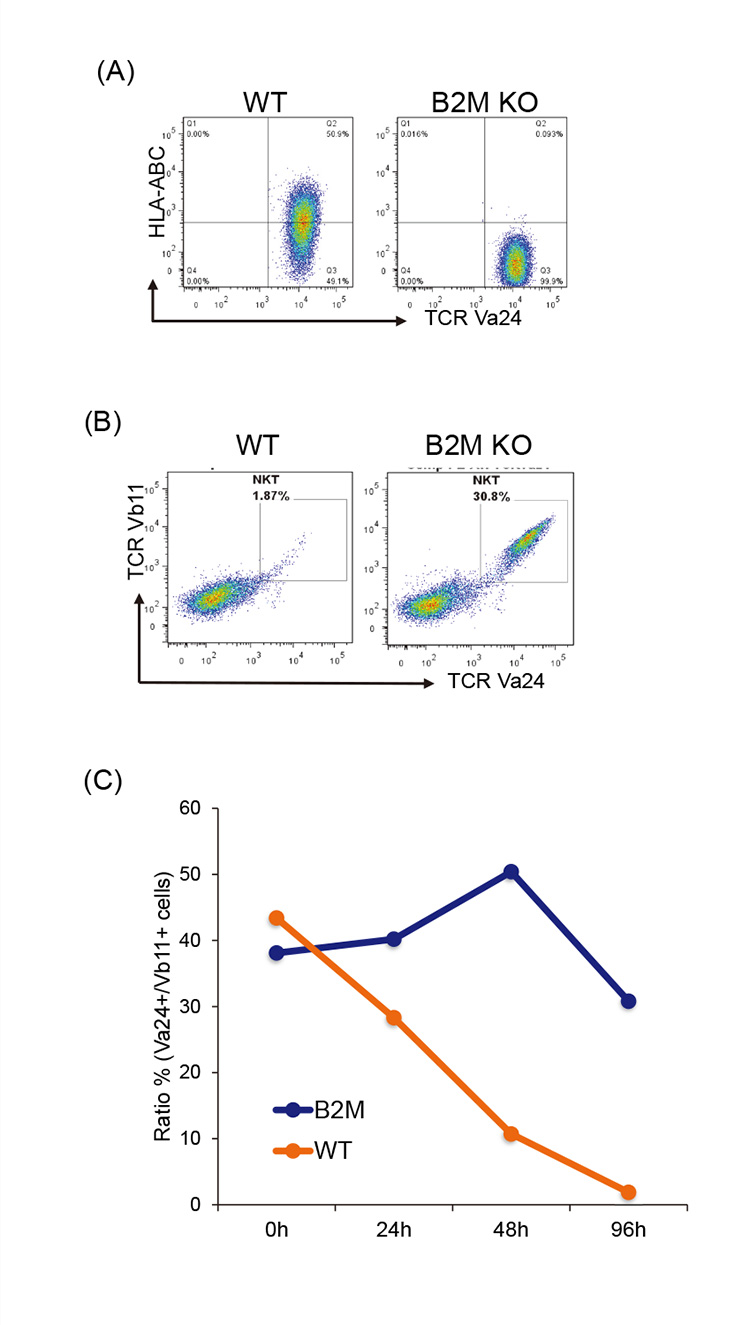
Figure: Minimization of alloreactive CD8+ T cell-mediated killing of HLA class I depleted iPS-Vα24+iNKT cells in vitro.
(A) A representative flow cytometry plot of B2M-disrupted iPS-Vα24+iNKT cells (B2M KO) showing lack of cell surface expression of MHC class I. Wild type (WT) iPS-Vα24+iNKT cells are shown as a control. (B) A representative flow cytometry plot of a cytotoxic assay of alloreactive CD8+ T cells against B2M KO or WT iPS-Vα24+iNKT cells after 96 hours. The alloreactive CD8+ T cell were prepared from healthy donor-derived PBMC and were cultured with B2M KO or WT iPS-Vα24+iNKT cells and analyzed by flow cytometry. (C) Time-course analysis of B2M KO or WT iPS-Vα24+iNKT cells in a cytotoxic assay of alloreactive CD8+ T cells. The frequency of the Vα24+iNKT cell specific TCR (Vα24+ and Vβ11+) cells was calculated at the indicated time points.