Laboratory for Inflammatory Regulation
Current research
The inflammatory response is an important host defense mechanism to sense and eliminate invading microbial pathogens. Dendritic cells first detect pathogens and activate the transcription factor NF-kB, which enters the nucleus and induces the expression of a series of inflammation-related genes. These initially helpful inflammatory responses must be terminated at the appropriate time point, otherwise; excessive responses can damage normal tissue and may cause autoimmune or allergic diseases. Our research goal is to identify key regulators of inflammation-related signal transduction pathways and to clarify the complete picture of the molecular mechanisms for regulating inflammatory responses. We previously identified PDLIM2 (PDZ and LIM domain protein-2), a nuclear protein that belongs to a large family of LIM proteins, as one of the key factors negatively regulating inflammatory responses. We found that PDLIM2 is an E3 ubiquitin ligase for the STAT4 and STAT3 transcription factors, thereby suppressing Th1 and Th17 cell differentiation (Tanaka T, Immunity, 2005, Tanaka T, Sci. Signal., 2011). We have also demonstrated that PDLIM2 negatively regulates NF-kB activity and subsequent inflammatory responses, acting as a nuclear ubiquitin E3 ligase targeting the p65 subunit of NF-kB. (Tanaka T, Nat. Immunol., 2007). We now focus on PDLIM2 and other members of the LIM family. Recently we found that PDLIM7 and PDLIM1 are also negative regulators of NF-kB-mediated signaling. PDLIM7 was found to be an E3 ubiquitin ligase for p65. PDLIM7 and PDLIM2 form heterodimers and synergistically promote p65 degradation by the proteasome. On the other hand, PDLIM1 sequestrated p65 in the cytoplasm, possibly by interaction with actin stress fibers, through binding to a-actinin and actin binding protein, and suppressed nuclear translocation of p65 protein. These studies should contribute to our understanding of the pathogenesis of human autoimmune and inflammatory diseases and the development of new therapeutic tools.
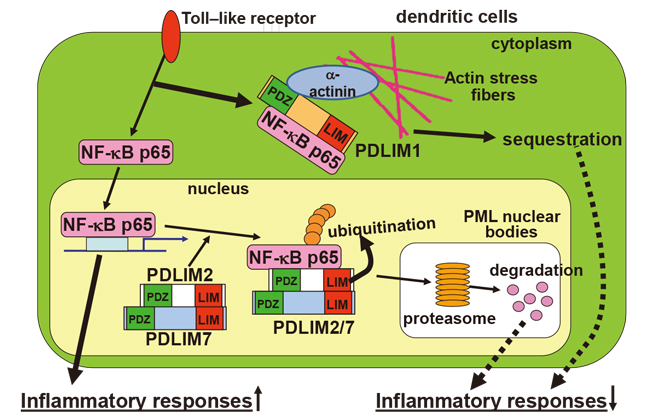
Figure: LIM is a new family of negative regulators of NF-kB signaling.
PDLIM2 and PDLIM7 are ubiquitin E3 ligases for the p65 subunit of NF-kB, form heterodimers and cooperatively promote p65 degradation. By contrast, PDLIM1 sequestrates p65 in the cytoplasm and inhibits its nuclear translocation, thereby suppressing NF-kB signaling.